Metformin Associated Lactic Acidosis
"The dose makes the poison"
Metformin a common medication used to treat DM II can cause metformin-associated lactic acidosis and Metformin toxicity with a mortality rate between 45-48%. Neither arterial lactate levels nor plasma metformin concentrations predicted mortality.
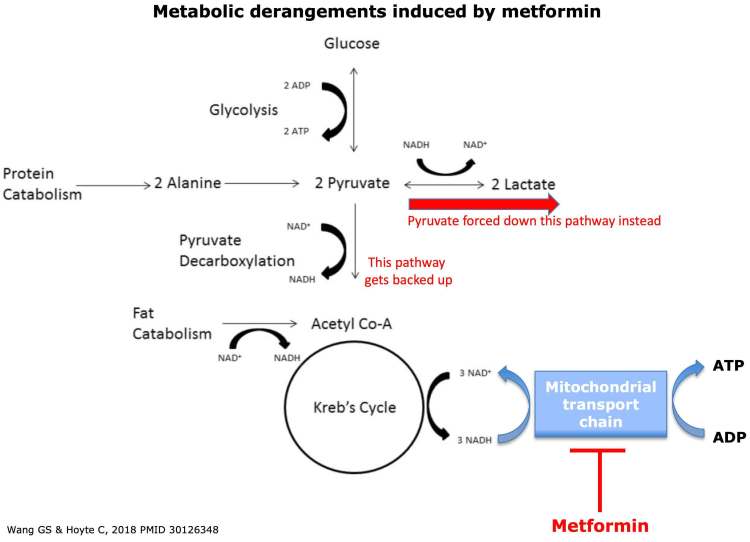
· The main effect of metformin is inhibition of the mitochondrial transport chain complex-I, which essentially poisons the mitochondria.
· If the mitochondrial transport chain stops working: NADH builds up, Krebs cycle eventually gets backed up, Pyruvate gets converted into lactate which builds up.
Best described a spectrum depending on cause of lactic acidosis
Metformin-induced lactic acidosis (MILA)
· High levels of metformin are the primary cause of illness.
Acute metformin overdose
· Acute poisoning may lead to MILA in the absence of renal dysfunction.
· Precise amount of metformin required to do this is unclear, but seems to be high (e.g. >20 grams).
· Patients with acute ingestion look fine initially, but deteriorate subsequently (“toxin bomb”).
Subacute accumulation of metformin due to renal failure
· Metformin is renally cleared
· Progressive renal failure (with GFR << 30 ml/min) eventually leads to metformin accumulation and toxicity.
· These patients may present with marked lactic acidosis, yet have fairly preserved hemodynamics and look OK.
Metformin-associated lactic acidosis (MALA)
Patient on metformin develops an acute life-threatening illness (e.g. septic shock, cardiogenic shock). Metformin amplifies the degree of lactic acidosis, but it's not the sole cause of the illness. Risk factors include renal insufficiency, higher doses of metformin, and alcoholism.
Metformin-unrelated lactic acidosis (MULA)
· Metformin levels are low; metformin is an innocent bystander.
· Clinically it will be impossible to differentiate this from MALA. Differentiation of MULA from MALA requires measurement of metformin levels, which isn't available at most hospitals.
Signs & symptoms
· Vitals: The following abnormalities may be seen: Hypothermia, Hypotension progressing to vasopressor-refractory shock can occur.
· GI symptoms often predominate: Nausea, vomiting, diarrhea, epigastric pain.
· Delirium, decreased consciousness
Management
· Fingerstick glucose (hypoglycemia may occur)
· VBG with lactate
· Complete set of chemistries (including Ca/Mg/Phos), Coags
· Beta-hydroxybutyrate level (frequently elevated)
· Liver function tests
· Blood cultures, urinalysis, chest X-ray, procalcitonin.
· Administer Broad-spectrum empiric antibiotics
· Additional toxicologic evaluation (e.g. acetaminophen, salicylate levels, toxic alcohols, carboxyhemoglobin).
· Obtaining a serum metformin concentration is unhelpful in most cases because few hospitals perform the test and thus timely results are rarely available, and because the serum concentration often does not correlate with the severity of the poisoning or patient outcome
· Obtain early consultation with a medical toxicologist and a nephrologist
Metformin-induced lactic acidosis vs. DKA
· Compared to isolated DKA, patients with metformin-induced lactic acidosis have greater degree of hyperlactatemia, with less extensive ketoacidosis.
· Difficult to sort this out in some situations- treat both conditions (the treatment for DKA may actually improve MILA/MALA).
· Activated charcoal may be considered for patients who present very shortly following acute ingestion, without contraindications (e.g. normal mental status without risk of aspiration)
· Evaluate for alternative causes of illness, especially septic shock.
· Insulin therapy may be beneficial for metformin poisoning (aside from any question of DKA) by reducing generation of lactate and ketosis thereby improving acidosis
Bicarbonate?
· Undesirable for a few reasons: Might increase cellular permeability to metformin, Bicarbonate has never been shown to be a useful therapy for lactic acidosis, Raising the pH with bicarbonate may actually stimulate glycolysis and thereby increase lactate generation
· Normal saline is an acidotic fluid that will exacerbate the acidosis.
· Lactated Ringers and Plasmalyte not good choice, as these patients cannot metabolize lactate or acetate respectively
Other options?
· D5W with 1/2 normal saline, plus one ampule (50 mEq) of bicarbonate added per liter.
· Simultaneous infusions of normal saline and isotonic bicarbonate
· High-flow nasal cannula may be used to improve ventilatory efficiency and reduce the work of breathing
Indications for dialysis
· Main indications
· Lactate >15-20 mM
· pH <7.0-7.1
· Failure to improve despite standard supportive measures
· Comorbid conditions which may lower the threshold for dialysis
· If the patient is hemodynamically unstable, CVVH or CVVHD should be considered. The clearance of drug by CVVH was less than that generally reported to occur with conventional hemodialysis and should only be considered in patients who are too hemodynamically unstable to tolerate hemodialysis.
Methylene Blue
Methylene blue is capable of accepting electrons from NADH could function as a bridge to re-establish the flow of electrons through the mitochondria and can theoretically re-starts the stalled Krebs cycle and re-establishes normal metabolism
Vasoconstriction: Methylene blue can also function as a vasoconstrictor (by scavenging nitric oxide). It's possible that its efficacy in some cases of refractory shock with metformin toxicity is due purely to its efficacy as a vasoconstrictor.
References:
UptoDate