FISH HOOK REMOVAL
Introduction
▪ Most fishhooks consist of an eyelet at one end, a straight shank, and a curved portion that ends in a barbed point on the inner curve that points away from the hook’s tip. By design, it is constructed to prevent the hook from dislodging once it engages tissue
▪ Fish hooks are most often caught on hands and feet
▪ ED physicians may remove superficially embedded hooks but those embedded in vital structures (eyes, testicles, carotid artery, etc) should be referred to the appropriate surgical specialist covering that organ
How do I prepare to remove it?
▪ Stabilize the hook with a hemostat and remove any attachments, such as lures, fishing lines, sinkers, etc.
▪ Cleanse with betadine
▪ Use local anesthesia
▪ Children may need procedural sedation
▪ Pain control
▪ Tetanus prophylaxis
What methods are used for removal?
▪ Back out technique
⁃ If the hook is barbless, this is the easiest method.
⁃ As the name implies, back the hook out with a hemostat.
▪ Push through technique
⁃ Use when the tip of the hook is near the skin surface.
⁃ Push the hook through until you break the skin, and then use a wire cutter to cut the tip off.
⁃ Then back out the remainder of the hook.
▪ String technique
⁃ Hook’s belly should be directly in front of you with the shank pointing in the opposite direction
⁃ Loop a piece of string or large silk suture (3-0) around the belly of the hook and then wrap the ends around your index finger
⁃ Push down on the shank and eye of the hook with your other hand to disengage the barb from the surrounding tissue
⁃ Pull string slowly until it is taut in the plane of the hook’s long axis
⁃ Keeping it taut, jerk it quickly and firmly in the same direction
▪ Cut it out technique
⁃ When all else fails, cut with a scalpel along the hook, and then blunt dissect down with a hemostat.
Should I give antibiotics?
▪ No trials have investigated antibiotic therapy for fish hook injuries
▪ Most superficial fish hook wounds heal well without sequelae
▪ Consider antibiotics if the fish hook is deeply embedded in an infection-prone area such as a fingertip or ear
▪ Most infections are caused by skin flora
▪ If hook is contaminated (touched sea water, fish, bait, etc), consider abx treatment
⁃ Cephalexin 500mg PO q6 or cefazolin 1g IV q8 or Clinda 300mg PO q6 or 600mg IV q8
⁃ Seawater? ADD Doxycycline 100mg q12
⁃ See recent guidelines for other specific situations
- POCUS
- Orthopedics
- Procedures
- Medications
- Pharmacology
- Respiratory / Pulm
- Infectious Disease
- Ophthalmology
- Airway
- Obstetrics / Gynecology
- Environmental
- Foreign Body
- Pediatrics
- Cardiovascular
- EKG
- Critical Care
- Radiology
- Emergency
- Admin
- Nerve Blocks
- DVT
- Finance
- EMS
- Benzodiazepines
- Neurology
- Medical Legal
- Psychiatry
- Anal Fissure
- Hemorroids
- Bupivacaine
- Ropivacaine
- EM
- Neck Trauma
- Emergency Medicine
- Maisonneuve Fracture
- Diverticulitis
- Corneal Foreign Body
- Gabapentin
- Lethal Analgesic Dyad
- Opioids
- Galea Laceration
- Dialysis Catheter
- Second Victim Syndrome
- Nasal Septal Hematoma
- Nephrology / Renal
- Hematology / Oncology
- Dental / ENT
- Dermatology
- Endocrine
- Gastroenterology
- October 2025
- September 2025
- August 2025
- July 2025
- May 2025
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- May 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
Trauma in Pregnancy
Resuscitation of the Pregnant Trauma patient
General principles
· Trauma is the most common cause of non-obstetrical maternal death in the United States
· Best fetal resuscitation is good maternal resuscitation.
· Stabilization of the pregnant women is the first priority; then, if the fetus is viable (≥ 23 weeks), fetal heart rate auscultation and fetal monitoring can be initiated and an obstetrical consultation obtained as soon as feasible
· In Rh-negative pregnant trauma patients, quantification of maternal–fetal hemorrhage by tests such as Kleihauer-Betke should be done to determine the need for additional doses of anti-D immunoglobulin.
· Tetanus vaccination is safe in pregnancy and should be given when indicated.
Airway
· Greater risk for difficult intubation than non-pregnant patient
· Pregnancy related changes à decreased functional residual capacity, reduced respiratory system compliance, increased airway resistance, and increased oxygen requirements
· Gastric emptying is delayed in pregnancy à greater risk for aspiration
· Respiratory tract mucosal edema à A smaller size of endotracheal tube is recommended
· Choice of RSI medications NOT affected by pregnancy status
Breathing
· Place chest tube one to 2 intercostal spaces higher than usual to account for displacement of the diaphragm during pregnancy
· Marked increases in basal oxygen consumption à lower threshold for supplemental oxygen
Circulation
· Fluid and Colloid resuscitation like standard trauma protocol
· Uteroplacental vasculature is highly responsive to vasopressors, and their administration may decrease placental perfusion à vasopressors should be avoided unless refractory
· Avoid supine hypotension: Compression of IVC by the uterus can cause up to 30% reduction in cardiac output à Place in left lateral position or by manual displacement of the uterus while the injured patient is secured in the supine position
· O-negative blood should be transfused in order to avoid Rh sensitization in Rh-negative women
· Vital signs: heart rate increases by 15% during pregnancy. Tachycardia and hypotension, typical of hypovolemic shock, may appear late in the pregnant trauma patient because of her increased blood volume.
· Maternal vital signs and perfusion may be preserved at the expense of uteroplacental perfusion, delaying the occurrence of signs of hypovolemic shock
· Attempt to obtain supra-diaphragmatic intravenous or intraosseous access for volume resuscitation and medication administration.
FAST
· The FAST is less sensitive for free fluid in the pregnant patient than in non-pregnant patients. Sensitivity decreases with increasing gestational age, likely due to altered fluid flow within the abdomen.
· Management of suspected placental abruption should not be delayed pending confirmation by ultrasonography as ultrasound is not a sensitive tool for its diagnosis.
Secondary survey
· In cases of vaginal bleeding at or after 23 weeks, speculum or digital vaginal examination should be deferred until placenta previa is excluded by a prior or current ultrasound scan.
Imaging
· Radiographic studies indicated for maternal evaluation including abdominal computed tomography should not be deferred or delayed due to concerns regarding fetal exposure to radiation.
· Ionizing radiation has the highest teratogenic potential during the period of organogenesis (5–10 weeks), with an increased risk of miscarriage before this period.
· With abdominal CT during the third trimester the fetal exposure is around 3.5 rads, which is still under the threshold for fetal damage
· Contrast agents should be used if indicated.
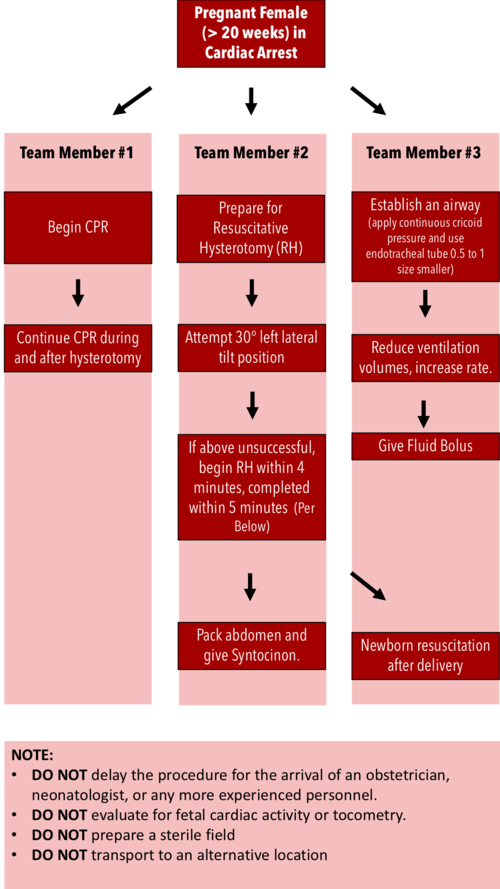
Resuscitative Hysterotomy in Cardiac Arrest
· Should begin within 4 minutes and completed within 5 minutes of cardiac arrest
· Both maternal and fetal survival decrease significantly after 5 minutes
· Do NOT delay the procedure for the arrival of an obstetrician or neonatologist.
· Do NOT evaluate for fetal cardiac activity or tocometry.
· Do NOT prepare a sterile field (but be as clean as possible).
· Do NOT transport to an alternative location.
· Performing RH increases maternal cardiac output by 30%.
References:
Jain, Venu, et al. "Guidelines for the management of a pregnant trauma patient." Journal of Obstetrics and Gynaecology Canada 37.6 (2015): 553-571.
Smith, Kurt A., and Suzanne Bryce. "Trauma in the pregnant patient: an evidence-based approach to management." Emergency medicine practice 15.4 (2013): 1-18.
Indications for use of Tranexamic Acid (TXA)
Indications for use of Tranexamic Acid (TXA)
Trauma
Trial Name: CRASH 2 (Positive trial)
Trial Type: Multicenter, double-blind RCT
Sample size: 20,211
Dose/Route of TXA: Loading dose 1g over 10 min, then infusion of 1g over 8hr
Primary outcome: All-cause mortality within 4 weeks of injury
Secondary outcome: Vascular occlusive events (AMI, stroke, PE, and DVT), surgical intervention, receipt of blood transfusion, and units of blood products transfused
Results: Reduced All-cause mortality p 0.0035, death due to hemorrhage p 0.0077, no significant vascular occlusion p 0.96
Risk of thrombotic events: No increase in risk
Take home point: The use of TXA in trauma patients with “significant bleeding” reduces all-cause mortality without an increase in thromboembolic events. This effect seems to be greatest in the subset of patients with severe shock (SBP ≤70mmHg) and when given ≤3 hours from time of injury
Shakur H et al. Effects of Tranexamic Acid on Death, Vascular Occlusive Events, and Blood Transfusion in Trauma Patients with Significant Haemorrhage. Lancet 2010. PMID: 20554319
Trial Name: MATTERs (Positive trial)
Trial Type: Single center, retrospective, observational study
Sample size: 896
Dose/ route of TXA: 1 g initially, 2nd dose per MD discretion
Primary outcome: 24hr mortality, 48hr mortality, and 30-day mortality
Secondary outcome: Transfusion requirements and rate of thromboembolic complications.
Results: Not significantly decreased 24 hr p >0.05, Significantly decreased 48hrs p 0.004 and 30 day mortality p 0.03
Risk of thrombotic events: Increased overall VTE p 0.001 but patients who had a VTE also had higher burden of injury
Take home point: Patients with penetrating injuries, requiring blood transfusions within 1hr of presentation the use of TXA reduced overall mortality
Morrison JJ et al. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg 2012. PMID: 22006852
ICH
Trial Name: Meta-Analysis of TXA for Traumatic Brain Injury- negative trial
Trial Type: Meta-analysis and systematic review of RCTs or quasi-RCTs
Sample size: 510
Outcome: Mortality, neurological function, hematoma expansion
Results: statistically significant reduction in ICH progression with TXA non-statistically significant improvement of clinical outcomes in ED patients with TBI.
Risk of thrombotic events: No adverse effects reported
Take home point: Did not lead to a statistically significant mortality benefit or improved neurological functional status. Further evidence is required to support its routine use in patients with TBI.
Zehtabchi S et al. Tranexamic Acid for Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Am J Emerg Med 2014. PMID: 25447601
Trial Name: Tranexamic Acid for Hyperacute Primary IntraCerebral Haemorrhage (TICH-2)- Negative
Trial Type: International, randomized, double-blind, placebo-controlled, parallel group
Sample size: 2325
Dose of TXA used: 1g IV TXA bolus followed by an 8hr infusion of 1g of TXA
Outcome: Functional Status at Day 90, Hematoma Expansion at Day 2, Mean Hematoma Volume Expansion from Baseline to 24hr, Death by Day 7, Death by Day 90
Results: No difference in neurological impairment (mean NIHSS score at day 7), 90-day functional outcomes, length of hospital stay, discharge disposition, venous thromboembolic events, or arterial occlusions
Risk of thrombotic events: None
Take home point: TXA was given >3hrs after stroke onset, patients had more severe strokes, and larger hematoma volumes (>60mLs) than prior studies. Possible benefit if given to a subset of patient within 3 hours with smaller strokes but cannot be recommended at this time in clinical practice for spontaneous ICH based on the results of these trials
Sprigg N et al. Tranexamic Acid for Hyperacute Primary IntraCerebral Haemorrhage (TICH-2): An International Randomised, Placebo-Controlled, Phase 3 Superiority Trial. Lancet 2018. PMID: 29778325
Post Partum Hemorrhage
Trial Name: WOMAN trial – Negative trial
Trial Type: Randomized, double-blind, placebo-controlled trial,
Sample size: 20,060 ≥16 years of age with post-partum hemorrhage after vaginal delivery or caesarean section
Dose of TXA used: 1 g IV vs matching placebo, If bleeding continued after 30 minutes or stopped and restarted within 24hrs, a second dose of 1g of TXA or placebo was given
Outcome: Initial outcome of all-cause mortality and/or hysterectomy within 42 days of giving birth
Final Primary Outcome: Death from PPH
Results: No difference in all cause mortality or hysterctomy
Risk of thrombotic events:
Take home point: It is difficult to draw definitive conclusions from this trial as the NNT was still large (i.e. ≈250) and the study had a fragility index of 0. Data showed a consistent association of delayed administration of TXA with no benefit
WOMAN Trial Collaborators. Effect of Early Tranexamic Acid Administration on Mortality, Hysterectomy, and Other Morbidities in Women with Post-Partum Haemorrhage (WOMAN): An International, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017. PMID: 28456509
UGIB
Trial Name: Cochrane review
Trial Type: Systematic review and meta-analysis of 8 RCTs
Sample size: 1700
Dose of TXA used: Total daily dose of TXA ranged from 4 – 8g and ranged from 2 – 7 days with both PO and IV adminsteration
Outcome: Primary: all-cause mortality and adverse events
Secondary: Rebleeding and surgery
Results: All-Cause Mortality p 0.007, rebleeding P = 0.07
Risk of thrombotic events: No difference in thromboembolic events (only evaluated in 4 trials)
Take home point: May benefit in higher risk patients but better RCTs required to confirm or refute evidence. HALT IT trial underway currently with N of 12000
Bennett C et al. Tranexamic Acid for Upper Gastrointestinal Bleeding (Review). Cochrane Database Syst Rev 2014. PMID: 25414987
Epistaxis
Trial Name: Zahed et al 2017 – Positive study
Trial Type: Randomized, parallel group clinical trial
Sample size: 124 on antiplatelets
Dose of TXA used: topical TXA (500mg in 5mL) or anterior nasal packing.
Outcome: Primary outcome resolution at 10 minutes. Secondary outcomes were re-bleeding rate at 24hours and one week, ED length of stay, and patient satisfaction
Results: epistaxis treatment with topical application of TXA resulted in faster bleeding cessation (NNT 2) , less re-bleeding at 1-week, shorter ED LOS, and higher patient satisfaction as compared with anterior nasal packing.
Risk of thrombotic events: not evaluated
Take home point: Do it!
Zahed R et al. Topical Tranexamic Acid Compared With Anterior Nasal Packing or Treatment of Epistaxis in Patients Taking Antiplatelet Drugs: Randomized Controlled Trial. Acad Emerg Med 2017. PMID: 29125679
Post-Tonsillectomy Bleeding
Trial Name: Meta-Analysis 2012
Trial Type: Systematic review and meta-analysis
Sample size: 7 studies with 2,444 patients
Dose of TXA used: 250mg for children <25kg, 500mg for children >25kg
Outcome: mean volume of blood loss
Results: TXA led to a significant reduction of tonsillectomy blood loss volume but had no impact on the rate of patients with post-tonsillectomy hemorrhage.
Risk of thrombotic events: Not evaluated
Take home point: In patients with minor post-tonsillectomy bleeding consider using nebulized TXA to reduce or stop bleeding.
Chan CC et al. Systematic Review and Meta-Analysis of the Use of Tranexamic Acid in Tonsillectomy. Eur Arch Otorhinolaryngol 2013. PMID: 22996082
Heavy Menstrual Bleeding
Trial Name: Cochrane Review
Trial Type: Systematic review and metanalysis
Sample size: 1312 in 13 RCTs
Dose of TXA used: majority of studies used regular dose TXA (ranging from 3 g/day to 4 g/day), Four other studies used low‐dose TXA (ranging from 2 g/day to 2.4 g/day)
Outcome: Volume of blood loss, Quality of life
Results: Appears effective for treating HMB compared to placebo, NSAIDs, Oral luteal progestogens, ethamsylate or herbal remedies but less effective than levonorgestrel intrauterine system
Risk of thrombotic events: Not studied in most RCTs
Take Home point: Antifibrinolytic treatment (such as TXA) appears effective for treating HMB compared to placebo, NSAIDs, oral luteal progestogens, ethamsylate, or herbal remedies. There were too few data for most comparisons to determine whether antifibrinolytics were associated with increased risk of adverse events, and most studies did not specifically include thromboembolism as an outcome.
Hemoptysis
Trial Name: Inhaled TXA RCT 2018
Trial Type: Prospective, double-blind, placebo-controlled randomized controlled trial
Sample size: 47
Dose of TXA used: nebulized TXA (500mg/5mL
Primary outcome: rate of complete resolution of hemoptysis during first 5 days from admission, difference in daily volume of expectorated blood
Secondary outcome: rate of interventional bronchoscopy, rate of angiographic embolization, rate of surgery, mean hospital LOS
Results: Resolution of hemoptysis within 5 days of admission, NNT = 2, P<0.0005. Statistically shorter LOS, less invasive procedures
Risk of thrombotic events: not studied
Take home point: Although this was a small study, the advantages of inhaled TXA vs placebo in patients with non-massive hemoptysis included faster resolution of hemoptysis, shorter hospital LOS, fewer invasive procedures, and although not statistically significant, a trend toward improved 30d mortality.
Wand O et al. Inhaled Tranexamic Acid for Hemoptysis Treatment: A Randomized Controlled Trial. Chest 2018. PMID: 30321510
References:
See above