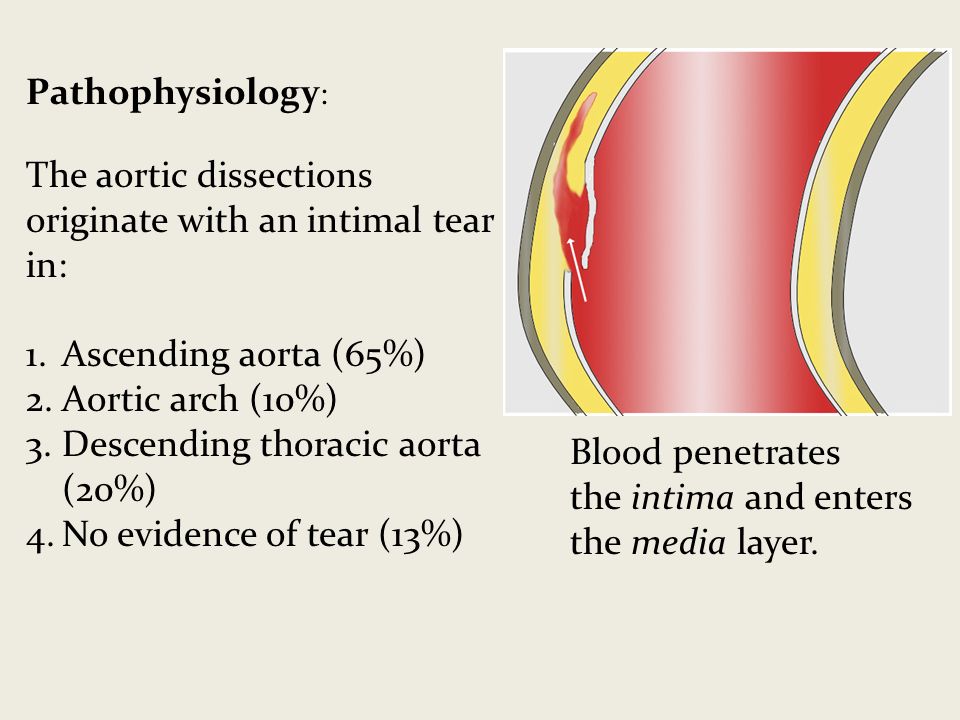
The following is a powerful advanced technique that can be used to troubleshoot traditional short-axis US-PIV placement.
A familiar scenario: The elusive needle tip!
You’re placing an US-guided PIV and going ahead with your short axis technique
The vessel is directly under the center of the probe, right on that Bx-guide line
You know exactly how deep it is
The needle has entered the skin… the tip should be right over or very near the vessel…
But where is it??? You’re bouncing the needle a little and see tissue moving, you’re slowly sweeping the probe backward and forward where your needle tip should be, but it continues to elude you! Maybe it’s a little deep, maybe there’s some echogenic (bright) tissue hiding it, doesn’t matter, here’s what to do...
The answer: Long Axis — Hear me out!
Take your eyes off of the ultrasound screen
Pick up the probe and place it back down, marker toward you, exactly along the axis of the angiocath, DIRECTLY over it!
Without moving your hand, look back up at the screen. Unless you’re at a crazy steep angle, you will see your whole needle clearly!
If you see the vessel on the screen as well, you are now perfectly set up to continue placing your IV
Position the tip in the vessel lumen, then advance the angiocath over the needle as you normally would

The other scenario: You’ve positioned the probe over the needle, you look up and see the needle but not the vessel any more, or maybe part of the vessel — here’s what you do
Slide/rotate the probe such that you have the vessel in view at its widest diameter on the screen
Then LOOK BACK AT THE ARM
If the probe is now to the right of the needle, you need to redirect to the right; if the probe is to the left, the needle needs to go left
Withdraw the needle a few mm and then redirect so that it is inline with the ultrasound
As you do this, look back up at the screen and you should see the needle coming into view
In a nutshell: If you’ve lost your needle tip
1. Use the probe to show you where the needle is
2. Use the probe to show you where the vessel is
3. With the probe over the vessel, position the needle so that it’s directly under the probe
4. Now all three are lined up and you’re ready to position the needle tip in the vessel lumen
A few last tips:
You can fine-tune your left-right control of needle tip in long axis by just moving the needle slightly one way or the other and seeing if it comes more into view or less into view — this will start happening automatically if you practice this technique a few times
I still recommend letting go of the probe and advancing the angiocath with non-dominant hand, however if an assistant takes the probe when you are ready to advance the angiocath, you can watch it go into the vessel and ensure that it is advancing smoothly into the lumen.
You can do this with one person as well but this requires advancing the angiocath and stabilizing the needle with one hand, which is more difficult and gives little tactile feedback as to whether it is advancing smoothly or meeting resistance
Once you’re comfortable with this long-axis technique, try doing the entire procedure in long axis. This tends to work very well for deeper, straighter veins.
There’s no reason you can’t switch back to short once you’ve found your needle tip and repositioned it; perhaps it’s a twisty vessel with multiple turns and you need to walk it in a little more - short axis is better for navigating in the left-right direction (as long as you’ve located your needle tip!)
Remember the concept of "angle of insonation": the steeper your needle angle, the more difficult it will be to see your needle because fewer ultrasound beams are bouncing back to the probe (more are being deflected in a different direction)
Jonas Pologe, PGY3, Emergency Medicine, Maimonides Medical Center